Cgmp Course
Cgmp Course - Learn more, view the details for cgmp online training: Ensure your product quality and regulatory compliance with cgmp certification. The ispe academy comprises eight distinct content areas: Using web based training, the course provides a breakdown of the current good manufacturing practice requirements from the fda and includes subpart detail. Four (4) free trial courses are available. A new generation, deviation investigations and investigation report writing, and gmp trainer certification. Also known as gmpro™, current good manufacturing practice (cgmp) professional certification program is an online training program that prepares and trains participants to be familiar with the latest cgmp regulations. Gmp, gamp®, plant engineering and maintenance, pharmaceutical technologies, regulatory, manufacturing operations, capital projects engineering, and personal development. Document your dedication to compliance, safety, and job performance by earning a professional certification. Document your dedication to cgmp compliance, consumer safety, and job performance by earning a professional certification from biopharma institute. Click here to request a quote. Encrypted pdf with validation qr barcode. Provides cgmp training in three categories; Four (4) free trial courses are available. Check out our on line training today. Learn more, view the details for cgmp online training: Document your dedication to compliance, safety, and job performance by earning a professional certification. The ispe academy comprises eight distinct content areas: A new generation, deviation investigations and investigation report writing, and gmp trainer certification. Document your dedication to cgmp compliance, consumer safety, and job performance by earning a professional certification from biopharma institute. Learn more, view the details for cgmp online training: Document your dedication to cgmp compliance, consumer safety, and job performance by earning a professional certification from biopharma institute. Learn its benefits, requirements, and steps to achieve this certification. Each cgmp certification training course is designed to present and explain cgmp mandates, as well as to provide comprehensive analysis and instruction. Check out our on line training today. Also known as gmpro™, current good manufacturing practice (cgmp) professional certification program is an online training program that prepares and trains participants to be familiar with the latest cgmp regulations. Gmp, gamp®, plant engineering and maintenance, pharmaceutical technologies, regulatory, manufacturing operations, capital projects engineering, and personal development. Document your dedication to cgmp compliance,. Gmp, gamp®, plant engineering and maintenance, pharmaceutical technologies, regulatory, manufacturing operations, capital projects engineering, and personal development. Document your dedication to cgmp compliance, consumer safety, and job performance by earning a professional certification from biopharma institute. A new generation, deviation investigations and investigation report writing, and gmp trainer certification. Encrypted pdf with validation qr barcode. Also known as gmpro™, current. The ispe academy comprises eight distinct content areas: Four (4) free trial courses are available. Learn its benefits, requirements, and steps to achieve this certification. A new generation, deviation investigations and investigation report writing, and gmp trainer certification. Using web based training, the course provides a breakdown of the current good manufacturing practice requirements from the fda and includes subpart. Encrypted pdf with validation qr barcode. Learn its benefits, requirements, and steps to achieve this certification. Four (4) free trial courses are available. Document your dedication to compliance, safety, and job performance by earning a professional certification. Gmp, gamp®, plant engineering and maintenance, pharmaceutical technologies, regulatory, manufacturing operations, capital projects engineering, and personal development. Learn its benefits, requirements, and steps to achieve this certification. Also known as gmpro™, current good manufacturing practice (cgmp) professional certification program is an online training program that prepares and trains participants to be familiar with the latest cgmp regulations. The ispe academy comprises eight distinct content areas: Four (4) free trial courses are available. Ensure your product quality and. Four (4) free trial courses are available. A new generation, deviation investigations and investigation report writing, and gmp trainer certification. Document your dedication to cgmp compliance, consumer safety, and job performance by earning a professional certification from biopharma institute. Provides cgmp training in three categories; Learn its benefits, requirements, and steps to achieve this certification. Ensure your product quality and regulatory compliance with cgmp certification. The ispe academy comprises eight distinct content areas: Encrypted pdf with validation qr barcode. Document your dedication to cgmp compliance, consumer safety, and job performance by earning a professional certification from biopharma institute. Also known as gmpro™, current good manufacturing practice (cgmp) professional certification program is an online training program. The ispe academy comprises eight distinct content areas: Encrypted pdf with validation qr barcode. Learn its benefits, requirements, and steps to achieve this certification. Click here to request a quote. Gmp, gamp®, plant engineering and maintenance, pharmaceutical technologies, regulatory, manufacturing operations, capital projects engineering, and personal development. Also known as gmpro™, current good manufacturing practice (cgmp) professional certification program is an online training program that prepares and trains participants to be familiar with the latest cgmp regulations. Provides cgmp training in three categories; Check out our on line training today. Ensure your product quality and regulatory compliance with cgmp certification. Learn more, view the details for cgmp. Encrypted pdf with validation qr barcode. Also known as gmpro™, current good manufacturing practice (cgmp) professional certification program is an online training program that prepares and trains participants to be familiar with the latest cgmp regulations. Click here to request a quote. Four (4) free trial courses are available. Using web based training, the course provides a breakdown of the current good manufacturing practice requirements from the fda and includes subpart detail. Document your dedication to compliance, safety, and job performance by earning a professional certification. Each cgmp certification training course is designed to present and explain cgmp mandates, as well as to provide comprehensive analysis and instruction on how to best comply. Learn its benefits, requirements, and steps to achieve this certification. A new generation, deviation investigations and investigation report writing, and gmp trainer certification. Check out our on line training today. Document your dedication to cgmp compliance, consumer safety, and job performance by earning a professional certification from biopharma institute. Learn more, view the details for cgmp online training:PPT FDA cGMP Training Program PowerPoint Presentation ID2946121
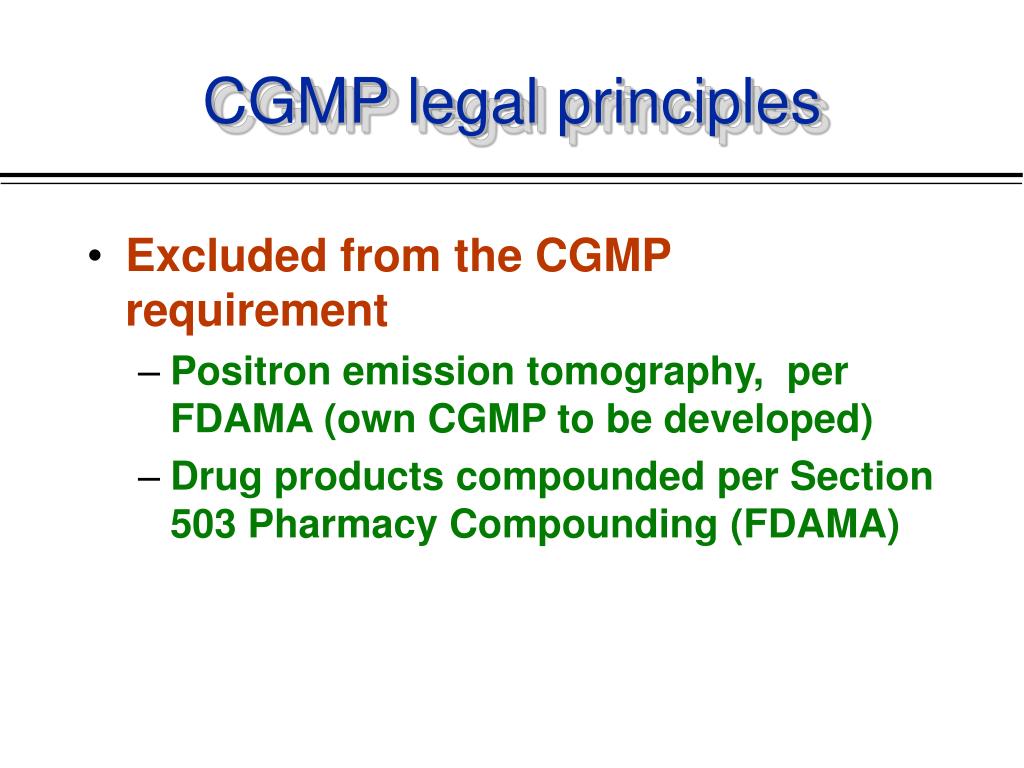
CGMP for Biopharmaceutical Drug Products Biotility
Introduction to Good Manufacturing Practices (cGMP) Training
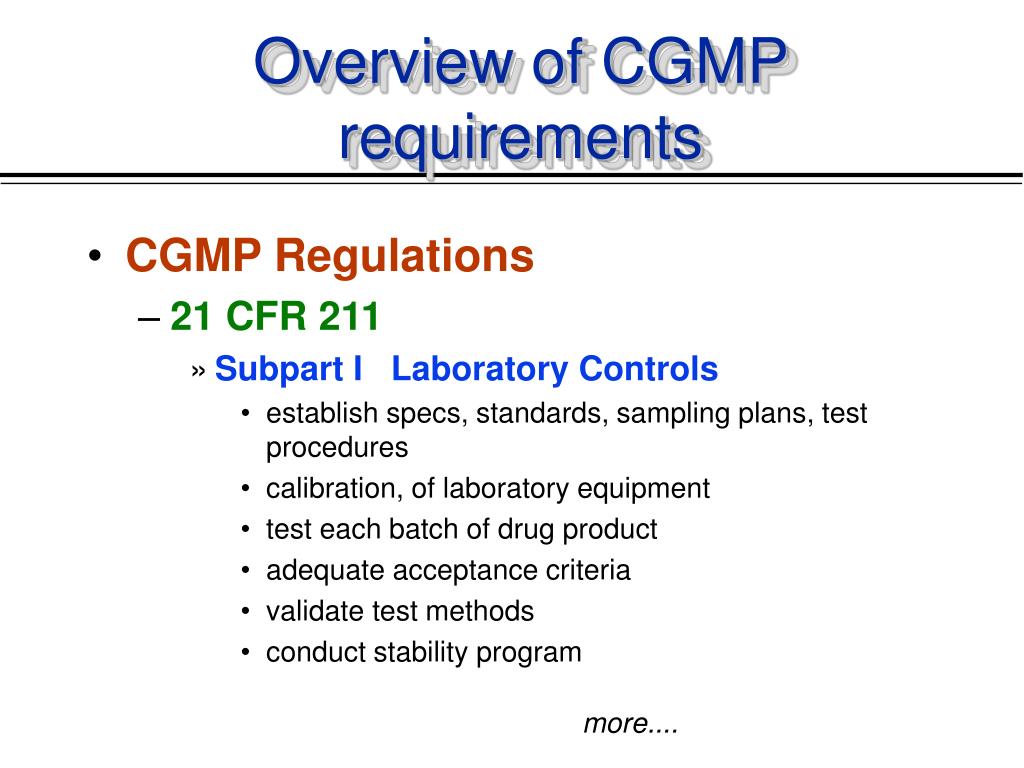
PPT FDA cGMP Training Program PowerPoint Presentation, free download
cGMP (Current Good Manufacturing Practice) Training Tonex Training Live
Quality Control Laboratory Compliance cGMP and GLP Training (Recorded)
Introduction To CGMP Compliance Course PDF Tablet (Pharmacy
cGMP Training Current Good Manufacturing Practices Online Training
PPT FDA cGMP Training Program PowerPoint Presentation, free download
PPT FDA cGMP Training Program PowerPoint Presentation, free download
Gmp, Gamp®, Plant Engineering And Maintenance, Pharmaceutical Technologies, Regulatory, Manufacturing Operations, Capital Projects Engineering, And Personal Development.
Ensure Your Product Quality And Regulatory Compliance With Cgmp Certification.
The Ispe Academy Comprises Eight Distinct Content Areas:
Provides Cgmp Training In Three Categories;
Related Post: