Iso 13485 Free Course
Iso 13485 Free Course - This course teaches all you need to know about iso 13485, helping you become an independent consultant for the standard. Iso 13485 is the cornerstone of quality management systems (qms) for medical devices. Free resources for iso 13485 implementation, auditing and training including tips on documentation requirements, required records, key links and articles, and more. Iso 13485 specifies qms requirements for the medical device manufacturing industry. Darüber hinaus lernen die teilnehmer mehr über die beziehung zwischen iso 13485 und iso 14971 „anwendung des risikomanagements auf medizinprodukte“. Der kurs behandelt die anforderungen der iso 13485:2016, indem er die wichtigsten grundsätze, die interaktion mit der iso 9001:2015 und mit den europäischen verordnungen (mdr und ivdr) erörtert. Alison offers free online engineering iso courses. Gain vital understanding of internationally recognized standards in engineering. You will study the iso 13485:2016 standard, learn how it was developed, and look into the practical steps to company certification on iso 13485. Recognize the benefits of an iso13485 qms for your organisation; Understand why iso 13485 quality management systems are important; Get started on your certification journey now. It means decisions are made free from any engagements of influences which could affect the objectivity of decision making. World’s most popular iso 13485 courses for beginners, and for experienced professionals. You will study the iso 13485:2016 standard, learn how it was developed, and look into the practical steps to company certification on iso 13485. Iso 13485 specifies qms requirements for the medical device manufacturing industry. This course teaches all you need to know about iso 13485, helping you become an independent consultant for the standard. Learn online for iso 9001, iso 27001, iso 14001, iso45001 and more. Choose from a range of free courses to enhance your knowledge of iso standards. If you’ve ever struggled to find iso 13485 training courses, webinars, online webinars and training materials like powerpoint presentations, this is a great site for you. Find iso 13485 training and lead auditor classes at asq.org. Iso 13485 is the cornerstone of quality management systems (qms) for medical devices. Iso 13485 specifies qms requirements for the medical device manufacturing industry. This free online iso 13485 certification training course will teach you about quality management systems for medical devices. Our free resources pages also provide information on. Find iso 13485 training and lead auditor classes at asq.org. This free iso 13485 internal auditor course will teach you everything you need to know about iso 13485 and how to perform an internal audit of your medical device quality management system (qms). Alison offers free online engineering iso courses. Upon completion of this course, you will be able to:. It means decisions are made free from any engagements of influences which could affect the objectivity of decision making. Gain vital understanding of internationally recognized standards in engineering. Enroll for free iso 9001 lead auditor You will need eight hours to complete the iso 13485 foundations course, 15 hours for the iso 13485 internal auditor course, and 20 hours for. This course is designed to provide you with a thorough foundation in the essential aspects of iso 13485:2016 standards, equipping you with the knowledge and skills needed to excel in quality management for medical devices. Upon completion of this course, you will be able to: All of the key iso 13485 and eu mdr documents, records, and templates necessary to. Learn all you need to know about a medical device qms in our iso 13485 foundations course for beginners. This free online iso 13485 certification training course will teach you about quality management systems for medical devices. Alison offers free online engineering iso courses. Free resources for iso 13485 implementation, auditing and training including tips on documentation requirements, required records,. Recognize the benefits of an iso13485 qms for your organisation; You will study the iso 13485:2016 standard, learn how it was developed, and look into the practical steps to company certification on iso 13485. Learn online for iso 9001, iso 27001, iso 14001, iso45001 and more. World’s most popular iso 13485 courses for beginners, and for experienced professionals. Enroll in. This training course explores the requirements of the iso 13485:2016 quality management system standard, discussing key principles and how the standard interacts with iso 9001:2015 and also it serves to facilitate global alignment for medical device quality management systems. Choose from a range of free courses to enhance your knowledge of iso standards. You will study the iso 13485:2016 standard,. Our free resources pages also provide information on how you can engage our experts, when needed. Describe why iso 13485 is important in helping ensure patient safety Find iso 13485 training and lead auditor classes at asq.org. Get started on your certification journey now. If you’ve ever struggled to find iso 13485 training courses, webinars, online webinars and training materials. You will need eight hours to complete the iso 13485 foundations course, 15 hours for the iso 13485 internal auditor course, and 20 hours for the iso 13485 lead auditor or lead implementer course. Der kurs behandelt die anforderungen der iso 13485:2016, indem er die wichtigsten grundsätze, die interaktion mit der iso 9001:2015 und mit den europäischen verordnungen (mdr und. Your certification costs will depend on the size of your business, location, and the sector you’re in. Iso 13485 specifies qms requirements for the medical device manufacturing industry. Upon completion of this course, you will be able to: Appreciate how your role is relevant to the iso13485 qms; If you’ve ever struggled to find iso 13485 training courses, webinars, online. World’s most popular iso 13485 courses for beginners, and for experienced professionals. Choose from a range of free courses to enhance your knowledge of iso standards. It means decisions are made free from any engagements of influences which could affect the objectivity of decision making. This free iso 13485 lead implementer course will teach you how to become an independent consultant or practitioner for the implementation of a medical device qms using the iso 13485 standard, and how to build your consultant business. Find iso 13485 training and lead auditor classes at asq.org. Your certification costs will depend on the size of your business, location, and the sector you’re in. Understand why iso 13485 quality management systems are important; Describe why iso 13485 is important in helping ensure patient safety Learn online for iso 9001, iso 27001, iso 14001, iso45001 and more. This free online iso 13485 certification training course will teach you about quality management systems for medical devices. You will need eight hours to complete the iso 13485 foundations course, 15 hours for the iso 13485 internal auditor course, and 20 hours for the iso 13485 lead auditor or lead implementer course. This free iso 13485 internal auditor course will teach you everything you need to know about iso 13485 and how to perform an internal audit of your medical device quality management system (qms). This course is designed to provide you with a thorough foundation in the essential aspects of iso 13485:2016 standards, equipping you with the knowledge and skills needed to excel in quality management for medical devices. If you’ve ever struggled to find iso 13485 training courses, webinars, online webinars and training materials like powerpoint presentations, this is a great site for you. Get started on your certification journey now. Free resources for iso 13485 implementation, auditing and training including tips on documentation requirements, required records, key links and articles, and more.ISO 13485 Online Training available 24/7 ISO 21001 accredited
Online Quality Management for Medical Devices and ISO 13485 Course
ISO 13485 Online Training available 24/7 ISO 21001 accredited
Free ISO 13485 Foundations Online Course Advisera Training
ISO 13485 Certification Training Courses
Free ISO 13485 Foundations Online Course Advisera Training
ISO 13485 Implementation Course JC Auditors Academy
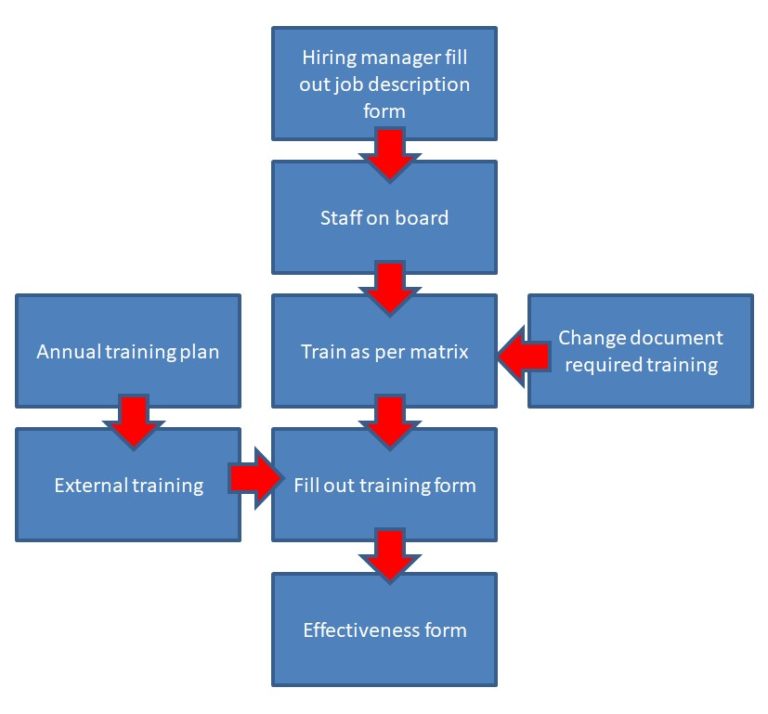
Free ISO 13485 Training Procedure Template
Free ISO 13485 Foundations Online Course Advisera Training
Free download of ISO 13485 & other medical device standards
Learn All You Need To Know About A Medical Device Qms In Our Iso 13485 Foundations Course For Beginners.
This Training Course Explores The Requirements Of The Iso 13485:2016 Quality Management System Standard, Discussing Key Principles And How The Standard Interacts With Iso 9001:2015 And Also It Serves To Facilitate Global Alignment For Medical Device Quality Management Systems.
Appreciate How Your Role Is Relevant To The Iso13485 Qms;
This Training Course Explores The Requirements Of The Iso 13485:2016 Quality Management System Standard, Discussing Key Principles And How The Standard Interacts With.
Related Post: